The Physics of Nuclear Power
What is Nuclear Power? - Fusion Vs Fission - Uses of Nuclear Power - Pros and Cons - Resources Used
Fusion Vs Fission?
Fission:
Fission is the splitting of large nuclei into smaller nuclei, resulting in smaller particles and energy being released. These smaller particles typically consist of other elements, neutrons and alpha, beta or gamma particles.There are two basic types of nuclear fission: 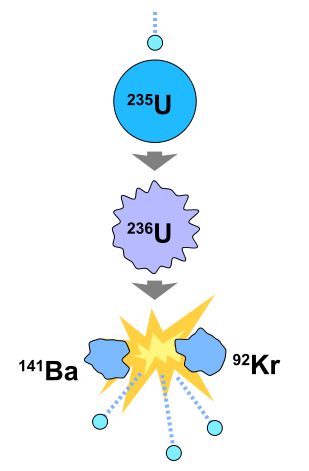
-
The first of these, spontaneous decay, is the random decay of unstable nuclei. This is seen in the decay of carbon-14, that is used to date archaelogical remains. It is also seen in the hands of older watchs that had hands that glowed due to the decay of radium.
-
The second type of nuclear fission, induced fission, is the result of the injection of a neutron in the nucleus of an atom. This injection causes the nucleus to become unstable and break down into smaller components. In the case of a sustanable nuclear reaction, this break down must include the expulsion of neutrons. Current nuclear power plants use this type of nuclear reaction. A good example of this is a common fuel for nuclear power plants, uranium-235. This process (As shown in the picture to the right) begins with the injection of a free neutron into the nucleus. At this point the uranium nucleus becomes unstable and breaks down into barium-141, krypton-92 and three free neutrons. These neutrons then go on to fuel this process for other uranium nuclei or are captured by the control rods in the reactor.
Fusion:
Fusion is the fusing of two small nuclei into one larger nuclei. This combination releases an enormous amount of energy. It is important to rememeber that every person on earth is intimiatly dependant on fusion reactions. This is because all of the energy that comes from the sun is a result the fusion of light elements. If you would like to know more about this subject, I recommend you email Dr.David Newman @ ffden@uaf.edu.